

If that happens, it will either be assisted by another chaperone and given time to fold successfully or it will be destroyed. For example, Spy releases clients in a largely folded state, while clients seem to be unfolded upon release from Trigger Factor or DnaK. Chaperone proteins are still proteins and they can certainly misfold just like any other. However, there are also key differences, especially in the details of how the chaperones release clients and how ATP cycling impacts that process.
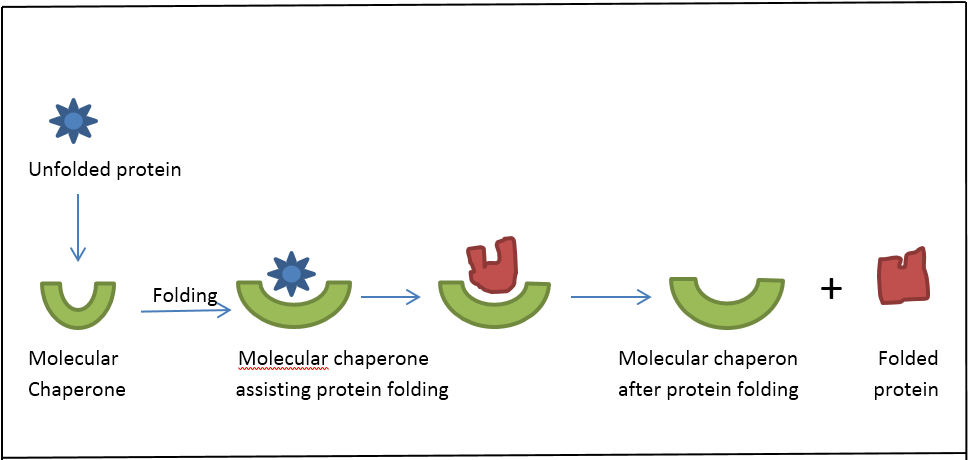
Thus, the relatively weak affinity of these interactions seems to provide directionality to the folding process. The underlying functional principles of the different chaperone classes are beginning to be understood. They share the ability to recognize and bind nonnative proteins thus preventing unspecific aggregation. Chaperone protien will help to refold if the protein can’t repair itself. Chaperones are a functionally related group of proteins assisting protein folding in the cell under physiological and stress conditions. The exist to ensure that proteins will form and have proper structure and function. They form an important line of defense against misfolded proteins and are part of the cellular quality control system. One striking similarity is that the chaperones all bind weakly to their clients, such that the chaperone-client interactions are readily outcompeted by stronger, intra- and intermolecular contacts in the folded state. The ribosome does protein synthesis, the chaperone protein is there to protect newly born protein until whole thing is made. Chaperones and co-chaperones regulate protein folding and client maturation, but they also target misfolded or aggregated proteins for refolding or for degradation, mostly by the proteasome. Here, we review examples from four distinct chaperones, Spy, Trigger Factor, DnaK, and HscA-HscB, highlighting the similarities and differences between their mechanisms. For the first time, recent advances in NMR spectroscopy have enabled detailed studies of how unfolded, "client" proteins interact with both ATP-dependent and ATP-independent classes of chaperones. Despite the central importance of this process to protein homeostasis, it has not been clear exactly how chaperones guide this process or whether the diverse families of chaperones use similar mechanisms. These considerations help to explain why cells must invest in an extensive network of factors, comprising 800 proteins in human cells ( 200 chaperones and co-chaperones and 600 UPS and. The major classes of molecular chaperones have highly variable sequences, sizes, and shapes, yet they all bind to unfolded proteins, limit their aggregation, and assist in their folding.


 0 kommentar(er)
0 kommentar(er)
